About the exam
SAT subject test are conducted to evaluate proficiency of student in a particular subject. Subject test are offered in Math, Literature, Science, etc.
Duration of SAT Chemistry Subject Test: 1 hour
Questions: 85 questions
Scaled Score Range: 200-800
The exam is offered in the months of August, October, November, December, May, and June.
- Calculators are not allowed.
- Measurements are expressed in the metric system.
- A periodic table is provided on the test.
Types of questions:
Type: 1
Matrix based questions; for example
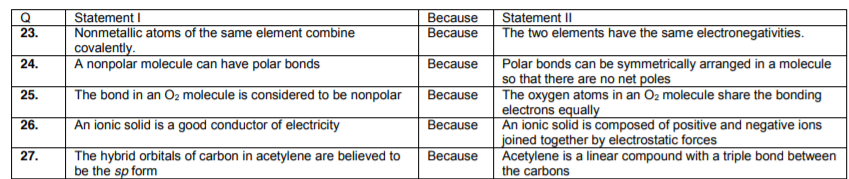
There are five choices for these question type. For each question you have to determine three things;
- Whether statement – 1 is true or false.
- Whether statement – 2 is true or false.
- Whether statement – 2 is correct explanation for statement – 1 or no.
Hence, we have 5 options;
TTCE – Statement – 1 and – 2 both are true and statement – 2 is correct explanation for statement – 1.
TTNCE – Statement – 1 and – 2 both are true and statement – 2 is not the correct explanation for statement – 1.
TF – Statement – 1 is true but statement – 2 false.
FT – Statement – 1 is false but statement – 2 true.
FF – Both statements are false.
Type: 2
Questions in group (same options for all of them); for example;

In these types of questions type, generally 3-5 questions are given in a group and for all of them we have same “five” answer choices.
In the above example 9, 10, and 11 are the questions and a, b, c, d and e are the option choices.
Good thing about this question type is that all questions are scored separately.
Type: 3
Normal MCQ questions with 5 answer choices. You have to select one option out of the five given options.

Course Structure:
| Skill-set and chapters to be covered | Expected questions |
| Structure of matter: Atomic Structure, including experimental evidence of atomic structure, quantum numbers and energy levels (orbitals), electron configurations, periodic trends. Molecular Structure, including Lewis structures, three-dimensional molecular shapes, polarity. Bonding, including ionic, covalent, and metallic bonds, relationships of bonding to properties and structures; inter molecular forces such as hydrogen bonding, dipole-dipole forces, dispersion (London) forces. | One can expect somewhere 25% of the total questions. |
| States of matter: Gases, including the kinetic molecular theory, gas law relationships, molar volumes, density, and stoichiometry. Liquids and Solids, including intermolecular forces in liquids and solids, types of solids, phase changes, and phase diagrams. Solutions, including molarity and percent by mass concentrations, solution preparation and stoichiometry, factors affecting solubility of solids, liquids, and gases, qualitative aspects of colligative properties. | One can expect somewhere 16% of the total questions. |
| Reaction types: Acids and Bases, including Brønsted-Lowry theory, strong and weak acids and bases, pH, titrations, indicators. Oxidation-Reduction, including recognition of oxidation-reduction reactions, combustion, oxidation numbers, use of activity series. Precipitation, including basic solubility rules | One can expect somewhere 14% of the total questions. |
| Stoichiometry: Mole Concept, including molar mass, Avogadro’s number, empirical and molecular formulas. Chemical Equations, including the balancing of equations, stoichiometric calculations, percent yield, and limiting reactants | One can expect somewhere 14% of the total questions. |
| Equilibrium and reaction rates: Equilibrium Systems, including factors affecting position of equilibrium (LeChâtelier’s principle) in gaseous and aqueous systems, equilibrium constants, and equilibrium expressions. Rates of Reactions, including factors affecting reaction rates, potential energy diagrams, activation energies | One can expect somewhere 5% of the total questions. |
| Thermochemistry Including conservation of energy, calorimetry and specific heats, enthalpy (heat) changes associated with phase changes and chemical reactions, heating and cooling curves, entropy | One can expect somewhere 6% of the total questions. |
| Descriptive chemistry Including common elements, nomenclature of ions and compounds, periodic trends in chemical and physical properties of the elements, reactivity of elements and prediction of products of chemical reactions, examples of simple organic compounds and compounds of environmental concern | One can expect somewhere 12% of the total questions. |
| Laboratory Including knowledge of laboratory equipment, measurements, procedures, observations, safety, calculations, data analysis, interpretation of graphical data, drawing conclusions from observations and data | One can expect somewhere 8% of the total questions. |
